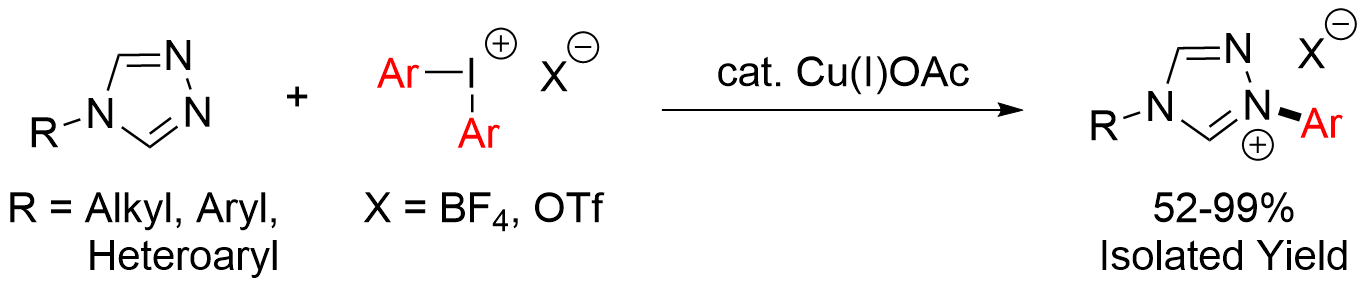Research
The Bolliger Group's research aims to establish novel methodology for the synthesis and functionalization of heterocycles.
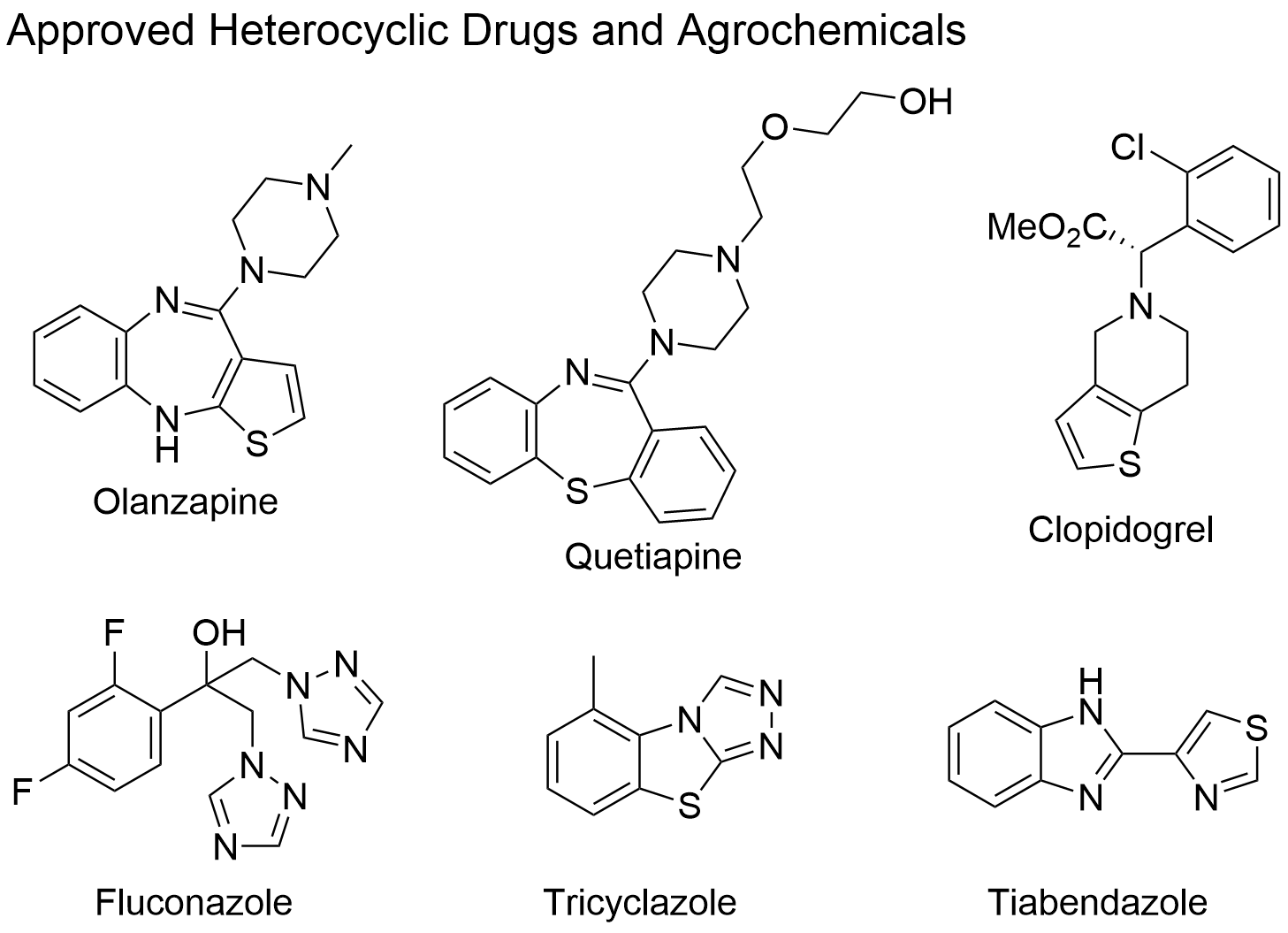
Heterocycles are ubiquitous in nature, ranging from alkaloids to the bases holding together the two strands our DNA through hydrogen bonding. Heterocyclic compounds are also of crucial importance to medicinal chemists as the majority of active pharmaceutical ingredients contain at least one heterocycle in their scaffold. For example, the antifungal drug fluconazole contains two 1,2,4-triazole rings. Although significantly less common then nitrogen- and oxygen-derived heterocycles, sulfur-containing heterocyclic pharmaceuticals exist. Examples of approved drugs incorporating both sulfur and nitrogen atoms in their cycles, are olanzapine and quetiapine (both antipsychotic drugs) and clopidogrel, an anticoagulant. Many sulfur-containing heterocyclic compounds also exhibit antimicrobial or antifungal properties such as the fungicide tricyclazole and the antifungal and antiparasitic agent tiabendazole. Since bi- and tricyclic systems containing a 1,2,4-triazoles joined with a sulfur heterocylce exhibit a wide range of biological activity including antimicrobial, antifungal, antioxidant, anti-inflammatory, and anticancer activity, much of the research in the Bolliger group focuses on developing new methods to prepare both known and novel fused heterocyclic scaffolds.
Synthesis of Tricyclic Heteroarenes by C–H Bond Functionalization
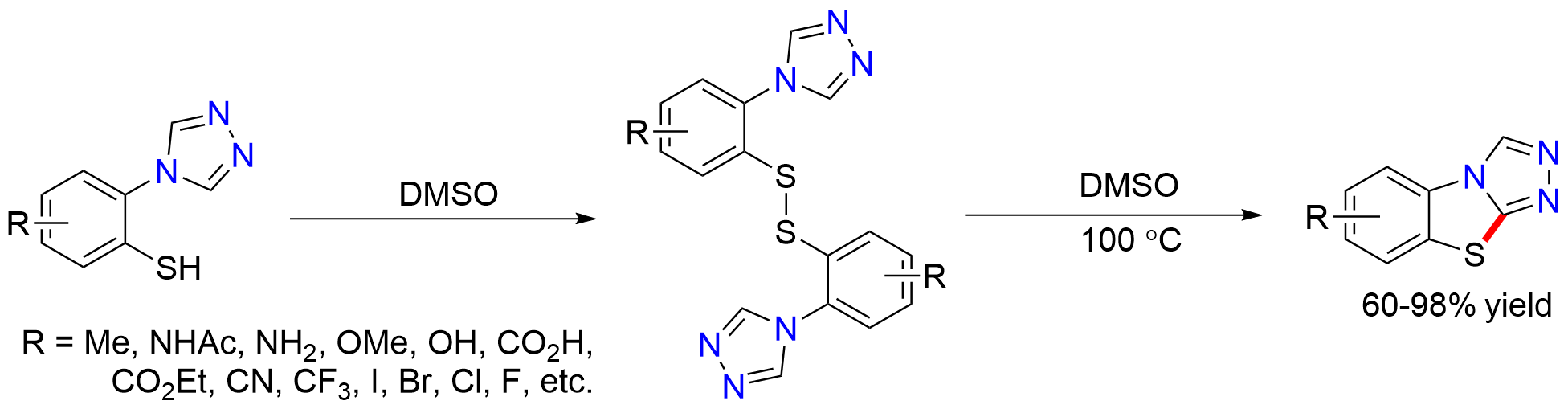
The Bolliger Lab is interested in developing new routes to neutral and charged heteroarenes. Among these heterocycles are benzo[4,5]thiazolo[2,3-c][1,2,4]triazoles for which two main synthetic approaches exist. Our new synthetic protocol allows the preparation of these tricyclic compounds via the oxidation of a mercaptophenyl moiety to its corresponding disulfide. Subsequent C–H bond functionalization is thought to enable an intramolecular ring closure, thus forming the desired benzo[4,5]thiazolo[2,3-c][1,2,4]triazole. This method combines a high functional group tolerance with short reaction times and good to excellent yields.
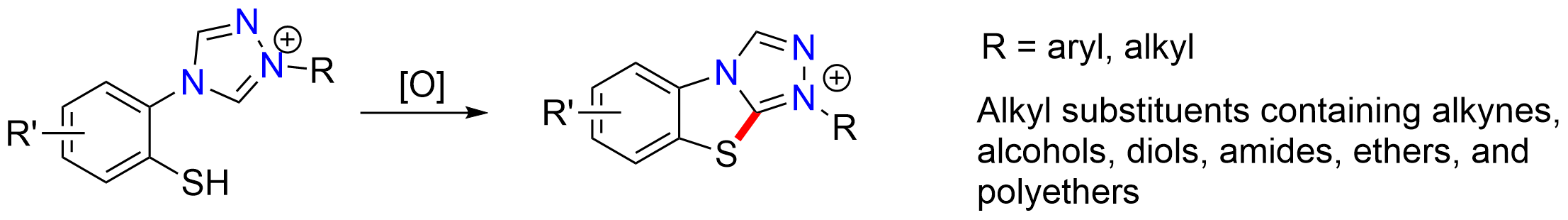
Using a similar procedure as with the neutral heteroarenes, we are able to extend the C–H bond functionalization approach to the synthesis of novel N-substituted benzo[4,5]thiazolo[2,3-c][1,2,4]triazol-1-ium salts from air stable precursors. This transformation is carried out via the selective deprotection of a para-methoxybenzyl protected thiol followed by C–H functionalization of the linked 1,2,4-triazolium salts under oxidative conditions, thereby forming the fused aromatic heterocycle. Using this procedure, we are able to synthesize a variety of tricyclic thiazolium salts which contain both electron-withdrawing and electron-donating aromatic substituents as well as aliphatic substituents. Our approach has proven to be tolerant towards many functional groups including alkynes, alcohols, diols, amides, and polyethers. Diverse substituents are also tolerated on the benzene ring.
Functionalization of Heterocycles by Transition Metal Catalysis
1,4-diaryl and 1-aryl-4-alkyl substituted 1,2,4-triazolium salts are convenient air-stable precursors to carbenes used both as organocatalysts or as ligands for transition metal complexes. Traditionally, they are prepared via a multistep synthetic pathway with the low-yielding formation of the triazolium ring occurring in the last step. We have developed an alternative two-step synthesis involving the conversion of a primary amine or aniline derivative to the corresponding 4-substituted triazole followed by a copper catalyzed arylation with diaryliodonium salts. This transition metal catalyzed arylation can be carried out under mild conditions in acetonitrile and is tolerant towards both water and oxygen. Additionally, the high functional group tolerance of the protocol described here gives easy access to triazolium salts containing heterocyclic substituents or sulfides.
A non-conclusive list of (other) areas of interest to the Bolliger Lab:
- Heterocyclic Chemistry
- Catalysis (Transition Metal and Supramolecular Catalysis)
- Dynamic Covalent Chemistry
- Medicinal Chemistry
- Mechanistic Investigations
- in situ NMR Investigations
- Electrochemistry